What is Digital Spatial Profiling?
Spatial Biology Q&A: Profiling the Tumor Microenvironment of Neuroblastoma using ChipCytometry™
.png?width=325&height=325&name=Margarida%20CAR%20T%20Cell%20Therapy%20Post%20(2).png)
Researcher Spotlight
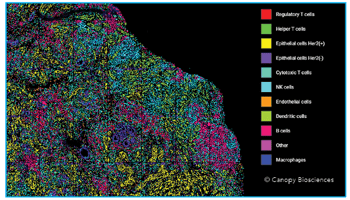
Profiling the Tumor Microenvironment of Neuroblastoma Using ChipCytometry™
Featuring Margarida Neves, MSc, PhD Student, Department of Pathology, UCL Cancer Institute, University College London Department of Translational Medicine, Autolus Limited
“We plan on utilizing the ChipCytometry platform and custom protein panels to continue to profile clinical samples. With CAR T-cell therapy specifically, we are interested in applying our discovered cell profiles to improve the design of new therapies.” — Margarida Neves, MSc
Tell us a little about yourself.
I am currently a PhD student with a joint appointment in the Department of Pathology at UCL Cancer Institute and the Department of Translational Medicine at Autolus Limited, co-supervised by Professor Teresa Marafioti and Dr. Mathieu Ferrari. We are studying CAR T-cell therapies which have the potential to deliver life-changing benefits to cancer patients.
What is your specific area of research interest or projects?
The goal of my PhD project is to analyze the complex interactions occurring in the tumor-immune microenvironment to ultimately inform the design of future CAR T-cell therapies. In doing so, I will evaluate existing open-source computational pipelines for their ability to assist with analysis of ChipCytometry datasets.
How have Canopy's products or services fit into your overall research goals?
We have used the ChipCytometry instrument to collect images of tissue biopsies from patients with neuroblastomas and other types of tumors. Luckily, the images can be exported as OME-TIFF files, which means that they are compatible with many open-source software.
How did working with Canopy scientists impact the quality of your experience?
It impacted my experience tremendously. Every time I had a query, Canopy scientists were quick to reply and solved the issue through troubleshooting. Overall, my experience using ChipCytometry instrument has been rewarding and it is exciting to work with this novel technique.
How did you analyze the multiple data types?
We have deployed various open-source tools for computational pathology to analyze our ChipCytometry datasets, including QuPath, Scanpy, and Squidpy. Additionally, we work with a bioinformatician who is developing new modules for our data analysis pipeline that will help us expand into new area
What aspects of this technology ultimately led you to choose ChipCytometry?
Because tissue-based ChipCytometry was initially developed for frozen tissue, there are more than enough pre-validated validated antibodies available for our experiments. Another reason why we chose the platform was because we needed a technology capable of true single-cell resolution in order to more accurately segment and phenotype cells in denser tissues.
How has using open-source reagents benefited your research?
The majority of our antibodies were previously validated for flow cytometry and available conjugated to a variety of dyes. Some of these dyes were readily compatible with the filter sets in the ChipCytometry instrument, so we were able to purchase these off-the-shelf without the need for custom conjugation.
Why is the ability to reinterrogate samples important for your studies?
The ability to reinterrogate samples has not yet been a critical aspect of our studies. We use a standard set of antibodies (i.e., a pre-defined antibody panel that has been decided prior to the start of staining) to image our samples. We expect this feature to be important in future studies.
Why is high dynamic range imaging important for your studies?
A differential expression analysis describes how protein expression varies across cellular profiles, which includes both highly abundant markers (i.e., CD45) and low abundance markers (i.e., PD-1). The benefit of high dynamic range (HDR) imaging is that we get a multi-exposure fused image to capture the lowest expressing proteins, which are typically out of reach for technologies that are more limited in sensitivity.
How does ChipCytometry fit as a complementary technique to other spatial technologies?
We also have experience with spatial transcriptomics using the NanoString Digital Spatial Profiler (DSP) platform to profile the same tissue specimens that we are profiling with ChipCytometry. Each of these platforms offers a unique set of information for complementary analyses.
What added value does analyzing both protein and RNA on the same tissue bring to your study?
We are interested in how the targets of our assay behave at both the transcriptomic and proteomic level, so we need to analyze both. We expect our analyses will yield interesting insights into the molecular mechanisms at play in these solid tumors.
For Research Use Only. Not for use in diagnostic procedures.